Inorganic chemistry is a branch of chemistry that deals with the structure, properties and reactivity of inorganic compounds.
- This article is an introduction to inorganic chemistry.
- We'll start by defining what inorganic chemistry is and comparing it to organic chemistry.
- We'll then look at some of the key ideas in inorganic chemistry.
- Finally, we'll provide an overview of some of the topics you can expect to cover in the following articles.
What are inorganic compounds?
Before we go any further, let's first define inorganic compounds.
Inorganic compounds are compounds that aren't based on carbon.
This might seem like a broad definition - it is! It encompasses all of the other elements in the world.
Take a look at the periodic table below. It shows carbon highlighted in pink. In inorganic chemistry, we look at compounds made from all of the other elements, from halogens to transition metals and everything in between.
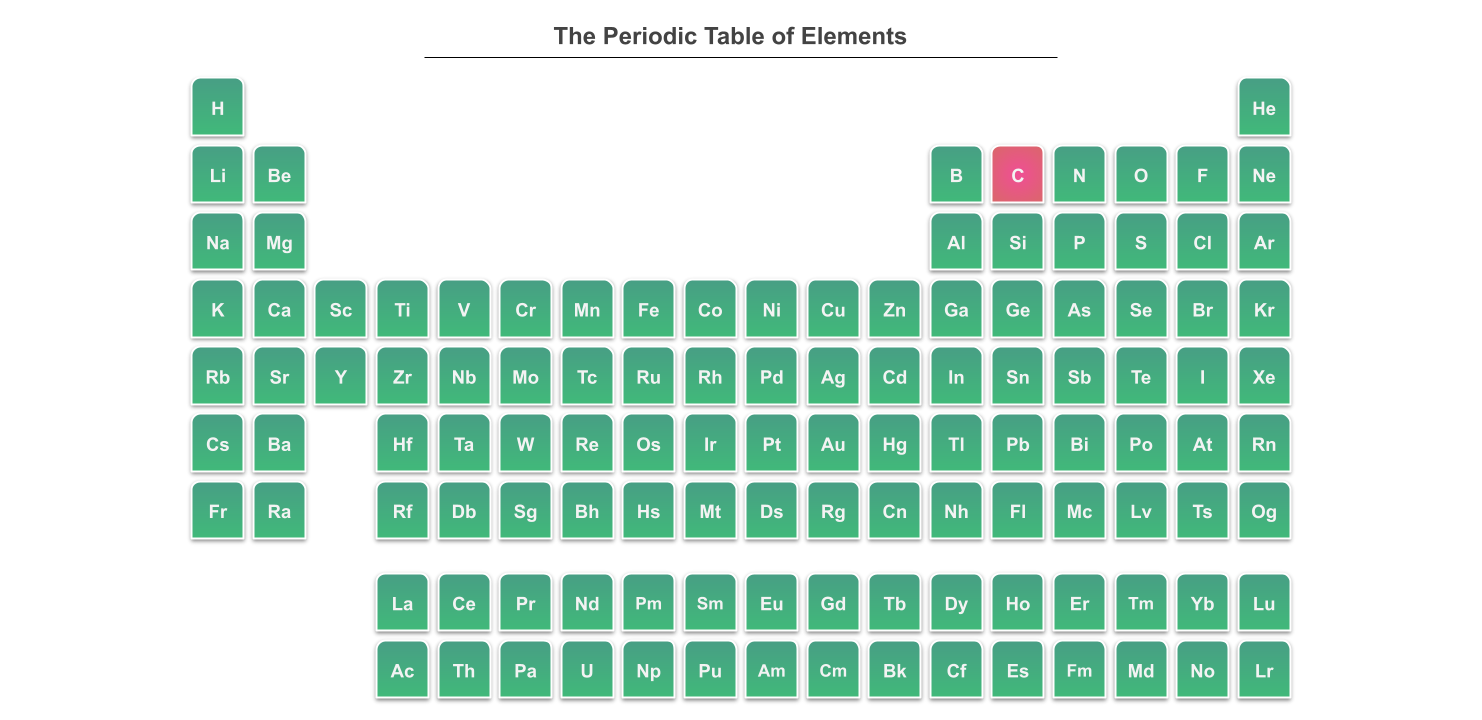 The periodic table. Inorganic chemistry focuses on compounds that aren't carbon-based. Here carbon is shown in pink. StudySmarter Originals
The periodic table. Inorganic chemistry focuses on compounds that aren't carbon-based. Here carbon is shown in pink. StudySmarter Originals
Inorganic chemistry doesn't ignore carbon - rather, it ignores carbon-based compounds. These are compounds that are based on CC and CH bonds. Such compounds are called organic compounds and you'll cover them in Organic Chemistry. They're so called because scientists originally believed that you could only find them in living organisms, but we now know that this isn't the case. But in inorganic chemistry, you'll find structures like graphite and diamond - both made of just carbon!
Going back to one of our examples at the beginning, the batteries in diesel and petrol cars are made up of electrodes placed in solution. The positive electrode, the anode, is coated in lead dioxide whilst the negative electrode, the cathode, is made from a grid of a lead alloy filled with sponge lead. The solution connecting the two electrodes is commonly sulfuric acid. This is known as the electrolyte. On the other hand, electric cars contain batteries with a graphite anode, a mixed-metal oxide cathode, and a lithium-ion electrolyte. You'll study all sorts of substances like these in inorganic chemistry. In fact, inorganic chemistry plays a vital role in many areas of life. For example, we use inorganic chemistry to design and develop things like catalysts, paints, batteries, surfactants, cleaners, jewellery, and drugs.
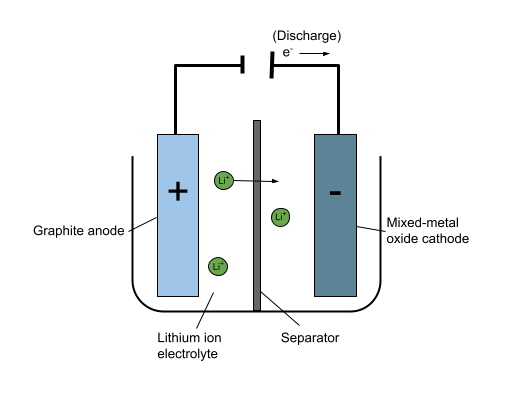 A simplified diagram of the battery in an electric car. Anna Brewer StudySmarter Originals
A simplified diagram of the battery in an electric car. Anna Brewer StudySmarter Originals
The basics of inorganic chemistry
Let's now look at some of the basic ideas you'll come across in inorganic chemistry.
The periodic table
The periodic table is a tabular arrangement of the chemical elements, organised by atomic number and properties.
The periodic table as we know it today is based on one created by Russian chemist Dmitri Mendeleev. He used knowledge about the properties of elements to order them in rows and columns, and even left gaps for undiscovered elements.
The periodic table:
- Has columns known as groups and rows known as periods. Some notable groups include the alkali metals (group 1) and the halogens (group 7, also known as group 17).
- Shows something called periodicity. This means that it contains patterns that repeat every row.
- Is organised into blocks.
- Contains metals, non-metals and metalloids. Metals are generally considered to be elements that lose electrons to form positive ions, whereas non-metals gain electrons to form negative ions. Metalloids behave somewhere between the two.
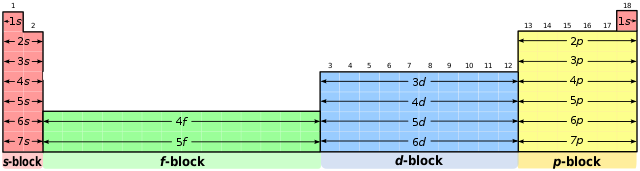 The periodic table split into four blocks,
The periodic table split into four blocks,
User:DePiep, CC BY-SA 3.0, via Wikimedia Commons
Ions
Ions are species formed when an atom loses or gains one or more electrons to form a charged particle.
Cations are positive ions whereas anions are negative ions.
Oxidation states
You might have seen species such as before. You can also find . What is the difference between the two?
Well, these species have different oxidation states.
Oxidation states show the total number of electrons which have been removed from an element (a positive oxidation state) or added to an element (a negative oxidation state) to get to its present state.
Oxidation states are very useful in redox reactions, which you'll look at below. We represent them using superscript numbers or roman numerals. For example, has an oxidation state of 2+ and can also be written as . This means that it has lost two electrons compared to a neutral iron atom.
Redox
Redox is a term we use to describe oxidation-reduction reactions, which you'll come across in physical chemistry. However, they are important in inorganic chemistry too.
Redox reactions are reactions where both oxidation and reduction take place. When a species is oxidised, it loses electrons, and when a species is reduced it gains electrons.
Lots of inorganic compounds are formed in redox reactions. Take a look at the example between zinc and copper sulfate:
We can show this as a redox reaction using oxidation states:
Note the following:
- Zinc is oxidised because it loses electrons.
- Copper is reduced because it gains electrons.
- Zinc acts as a reducing agent because it reduces copper.
- Copper acts as an oxidising agent because it oxidises zinc.
Acids and bases
You also learn about acids and bases in physical chemistry, but they are relevant here as well.
An acid is a proton donor whilst a base is a proton acceptor.
Some elements and compounds are much better acids or bases than others, and you'll learn a bit more about that in inorganic chemistry.
Transition states
A transition state is a stage of a reaction where some bonds are partly broken and some bonds are partly formed. At this point, the molecules are at their maximum energy level, making transition states extremely unstable.
Imagine running a reaction in slow motion and taking a picture halfway through. If you zoom in closely, you might see that some of the original bonds in the reactants have broken but new bonds haven't quite formed, or that intermediate compounds have formed instead. This is an example of a transition state.
Not all molecules that start reacting together go on to finish the reaction. At the transition state, there is an exactly 50 percent chance of the reaction finishing. Transition state theory tells us that once a reaction has passed transition state, it will always go on to completion.
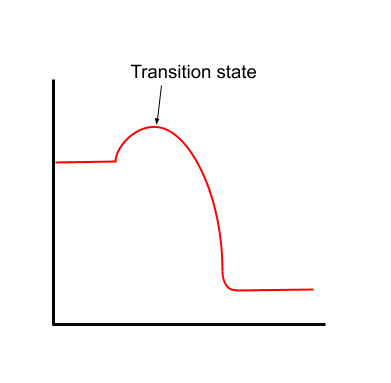 The transition state of a reaction. Anna Brewer, StudySmarter Originals
The transition state of a reaction. Anna Brewer, StudySmarter Originals
Types of compound
Organic chemistry is filled with molecules like alkenes, alcohols and amines. But in inorganic chemistry, you are much more likely to find salts, oxides and metalloids:
- A salt is an ionic compound formed when a positive ion bonds to a negative ion using electrostatic attraction. You might have come across salts before in physical chemistry, under Ionic Bonding.
- A mineral is a naturally occurring inorganic solid with a definite chemical composition and a crystalline structure. This means it contains a regular repeating arrangement of atoms.
- Oxides are compounds containing at least one oxygen ion with an oxidation state of 2+
- Nitrates are compounds containing the ion.
- Sulphates are compounds containing the ion.
- Organometallic compounds cross the bridge between organic and inorganic chemistry. They are compounds containing at least one bond between a carbon atom in an organic compound, and a metal or metalloid.
Topics within inorganic chemistry
In inorganic chemistry, you’ll study a variety of topics, ranging from periodicity and group 2 metals to halogens and ions. Let’s explore some of them below.
Periodicity and trends
As we mentioned earlier, the periodic table shows periodicity: it contains patterns that repeat across every row in the table. This means that as you travel down a column in the periodic table, known as a group, you’ll find that the elements all react in a similar way. (Look at Periodicity and Trends to explore some of the trends in the periodic table, including atomic radii and ionisation energy.) Knowing these makes it a lot simpler to predict how an element will react. You’ll focus specifically on elements in period 3.
Groups 2 and 7
Group 2 contains the alkaline earth metals whilst group 7 (also known as group 17) contains the halogens, a family of nonmetals. In these two topics, you’ll explore their chemical and physical properties. For example:
- How does reactivity change as you move down the elements in group 2?
- Which group 2 compounds are soluble and which are not?
- Which halogen has the best oxidising ability?
- What are some uses of chlorine?
Transition metals
After that, you’ll visit transition metals.
A transition metal is an element that forms ions with a partially-filled d subshell.
Most transition metals lie in the d block in the periodic table, but not all. Not all d block elements are transition metals either. For example, zinc only forms Zn2+ ions with a full 3d subshell, 3d10, and so isn’t a transition metal. You might also want to note that f block elements are considered to be transition metals. They’re often known as inner transition metals. In the periodic table below, we’ve shown the f block elements in purple and the d block elements in blue, with the transition metals within the d block circled in red:
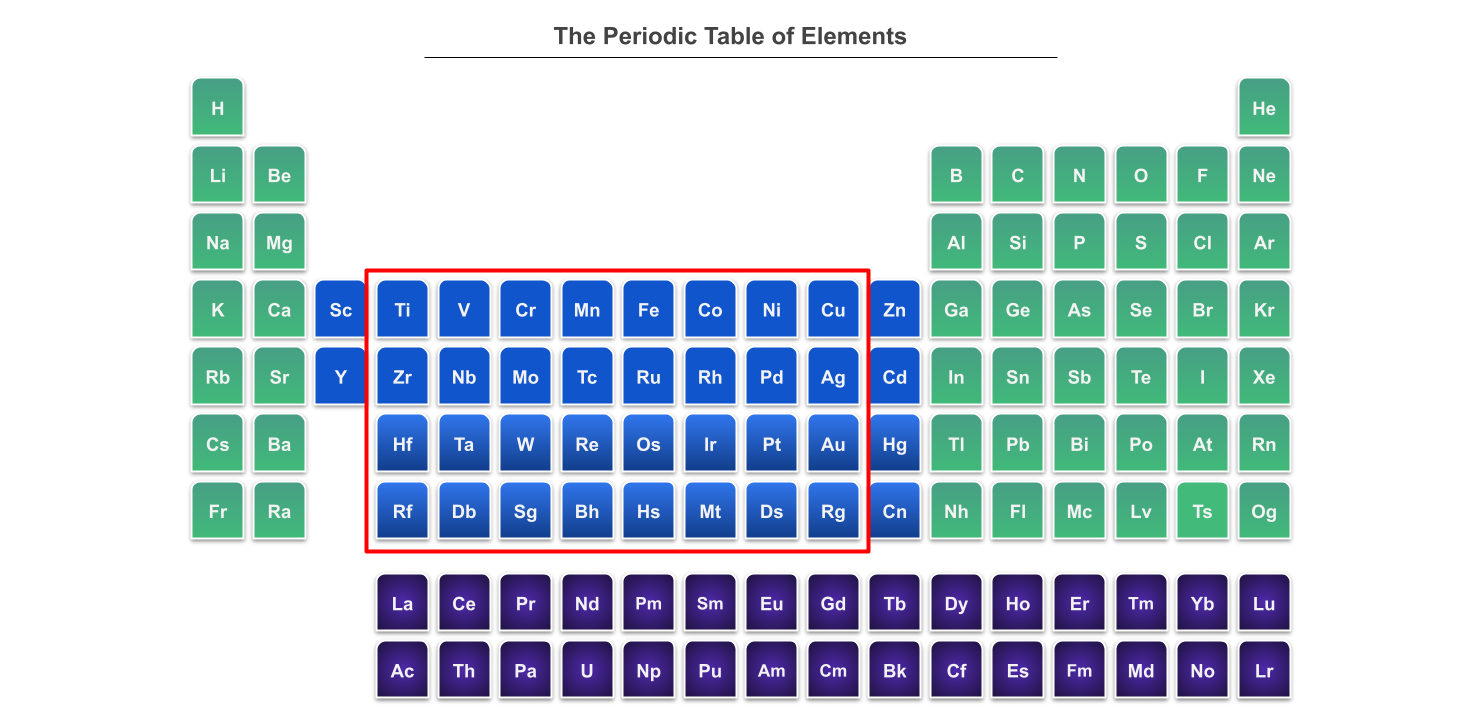 The periodic table, with the elements in the d and f blocks highlighted. StudySmarter Originals
The periodic table, with the elements in the d and f blocks highlighted. StudySmarter Originals
Transition metals share four common properties:
- They have variable oxidation states.
- They are brightly coloured.
- They are good catalysts.
- They form complex ions. A complex ion is a compound formed when a transition metal bonds to other species, known as ligands, using coordinate or dative covalent bonds.
You’ll learn all about these in Transition Metals.
Reactions of ions in aqueous solution
Finally, in this topic you’ll delve deeper into ions and acidity.
- Why are some metals better acids than others?
- What is chelation?
- How is water replaced in ligand substitution reactions?
Further topics in inorganic chemistry
Inorganic chemistry doesn’t stop at the topics we explored above. Further examples include the group 1 metals, known as alkali metals, electrolysis, group 4, and the extraction of metals.
Make sure to find out what your exam board wants you to know for inorganic chemistry. Not all exam boards will test you on every topic - although learning more is never a bad thing!
Inorganic Chemistry - Key takeaways
- Inorganic chemistry is a branch of chemistry that deals with the structure, properties and reactivity of inorganic compounds. Inorganic compounds are compounds that aren’t based on C-C and C-H bonds.
- Inorganic chemistry uses your knowledge of topics such as redox and acidity.
- In Periodicity, you’ll explore trends across the periodic table.
- In Group 2 and Group 7, you'll explore the properties and reactions of specific groups within the periodic table.
- In Transition Metals, you'll look at the properties of transition metals.
- In Transition Metal Ions in Aqueous Solution, you'll find out more about acidity and ligands.
- Other topics within inorganic chemistry include group 1 metals, group 4 elements, and electrolysis.
References
- The periodic table split into four blocks, shared under CC BY-SA 3.0(https://creativecommons.org/licenses/by-sa/3.0/)
Learn with 471 Inorganic Chemistry flashcards in the free StudySmarter app
We have 14,000 flashcards about Dynamic Landscapes.
Already have an account? Log in
Frequently Asked Questions about Inorganic Chemistry
What is the difference between organic and inorganic chemistry?
Organic chemistry is a field that studies the structure, reactivity and properties of carbon-based molecules. In contrast, inorganic chemistry studies the structure, reactivity and properties of compounds that aren’t based on carbon.
Why is inorganic chemistry important?
Inorganic chemistry plays a role in many areas of life. For example, we use inorganic chemistry to design and develop things like catalysts, paints, batteries, surfactants, cleaners, jewellery and drugs.
What does inorganic mean in chemistry?
Inorganic means not based on carbon. Whilst organic molecules are based around C-C and C-H bonds, inorganic compounds are based around all the other elements in the periodic table. Examples include salts and minerals.
What are some examples of inorganic chemistry?
Examples of inorganic compounds include salts, minerals, acids and metals. Examples of applications of inorganic chemistry include designing drugs, batteries, electronics and cleaning products.
Do mechanisms appear in inorganic chemistry?
Mechanisms do appear in inorganic chemistry but you won’t encounter them at this level of study.

About StudySmarter
StudySmarter is a globally recognized educational technology company, offering a holistic learning platform designed for students of all ages and educational levels. Our platform provides learning support for a wide range of subjects, including STEM, Social Sciences, and Languages and also helps students to successfully master various tests and exams worldwide, such as GCSE, A Level, SAT, ACT, Abitur, and more. We offer an extensive library of learning materials, including interactive flashcards, comprehensive textbook solutions, and detailed explanations. The cutting-edge technology and tools we provide help students create their own learning materials. StudySmarter’s content is not only expert-verified but also regularly updated to ensure accuracy and relevance.
Learn more